by Jack Brodie, James Hitchmough, and Michael Livingstone
There is a crisis in the UK, and many other developed countries, in the decline of a wide range of insects that visit flowers to gather nectar and pollen. The Twentieth Century saw major population declines, range contractions, and local extinctions of many wild insect pollinators such as wild bees and butterflies and managed honey bee populations. This has led to fears that the pollination services provided by insect pollinators, may be in danger of collapse. Indeed, there is suggestion that the feed-back processes behind this may already be occurring (Biesmeijer et al. 2006). The disappearance of pollinators would have dire consequences for agricultural crops, many wild plants, and ecosystems.

The near-native Origanum laevigatum (marjoram) is one of the most popular and very nectar rich plants in our study at the University of Sheffield. Photo: ©Jack Brodie
The decline of pollinators is due to a number of factors, but the single most important is the greatly reduced number of flowers in the landscape (Roulston & Goodall 2010). This is mainly due to the “green revolution” of intensive agriculture in the twentieth century. Many flower-rich habitats such as unimproved grassland, hay meadows, fallow fields, leguminous forage, and hedgerows were lost during this period. Lost habitat greatly reduced both the abundance and diversity of floral resources available in the landscape creating flower shortages in both space and time (Baude et al. 2016).
Ironically, given our historical beliefs that nature predominantly resides in the countryside, it is arable landscapes in which pollinators seem to have fared the worst, whilst urban areas have retained surprisingly resilient pollinator populations over this period (Senipathi et al. 2015) One recent study by Baldock et al (2015) even found UK urban areas to support greater bee diversity than adjacent farmland.
This suggests that designed landscapes can and do support pollinators, and that we as landscape architects can do a great deal to maintain pollinator diversity. Historically we have tended to see planting as either primarily for nature, or primarily for people. This is a redundant way to see planting design in twenty-first century cities; rather what we should be doing is to see all projects and sites as potential opportunities to provide pollinator services irrespective of where these sit on the nature-culture spectrum. So what are some of the key factors we need to consider in designing these plantings?
The Geographic Origins of Species
The belief that only plants that are native to the nation-state in question can effectively support native pollinators is widespread. However, the very fact that pollinators have fared so well in urban parks and gardens where most of the flowers are non-natives suggests that this idea is deeply flawed. This notion of the native superiority has more recently been bolstered by studies by the likes of Comba et al. (1999) who found that plant cultivars with double petals tend to have reduced nectar rewards and hence attractiveness to pollinators. This then became conflated with the spurious idea that most non-native species in the horticultural flora are similarly transformed and hence are no good for pollinators.
Some native species are excellent for pollinators and some are much less so; similar patterns are seen in non-native species. The non-native species that are least good are often those that have evolved to accommodate pollinators very different to our own native ones. For example Penstemon barbatus and Salvia splendens are both adapted to hummingbirds that are absent in western Europe. In ecological terms species can be thought of in relation to the UK as “native,” “near-native,” and “non-native.” “Near native” plants are species such as Eryngium planum that are not native to, in our case, the UK, but might be found, for example, on the western European mainland. “Non-native” plants are more geographically distant still.
Nearly all UK “native” plants and pollinators are distributed in other countries beyond the UK; we have virtually no UK endemics. For us these species are natives, but they are also native to distant places. Galium verum (ladies bedstraw) and Geranium pretense (meadow Cranesbill), for example, naturally extend from the UK all the way to Mongolia, while the white-tailed bumble bee naturally ranges from North America to Japan. Many of our native pollinators or insects very similar to them will have naturally co-existed and co-evolved with many “near-native” or even some “non-native” plant species beyond our borders and are therefore very likely to recognize these types of plant species as a resource. However, in many cases “non-native” plants are assumed not to have co-evolved with pollinators native to the UK, and hence in this situation are less likely to be of value to these. Clearly there are lots of ifs and buts in this. It’s a minefield of dubious assumptions that are only just beginning to be tested.
The good news is that there is a growing body of scientifically rigorous literature that quantifies the relative value of native and non-natives plant species to UK pollinators. What this data shows is that being valuable to pollinators is primarily dependent on the possession of certain biological characteristics or traits, which do not correlate neatly with original geographical distribution.
Recent studies by Hicks et al. (2016) and Garbuzov & Ratnieks (2014) have confirmed that non-native plants provide abundant and potentially very valuable resources. Salisbury et al. (2015) and Hanley et al. (2014) have shown that native pollinators are just as happy, and sometimes even prefer to utilize these non-native flowers when available. We have ourselves found in our Sheffield trials, strong evidence for the ecological value of non-native plants and particularly near-natives. As our climate warms and our “native” pollinator assemblage changes (yes, some currently non-native pollinators will become new “natives”), some near-native and non-native plants will potentially become more valuable. Combined with the usefulness of non-native species in other aspects such as flowering season, aesthetic appeal, and cultural importance, they should not be excluded from urban planting projects simply by virtue of their origin.
Plant species should instead be valued for the specific traits and characteristics which make them demonstrably valuable to pollinators.
Value to Pollinators –
Important Plant Characteristics and Traits
Nectar Rewards
The sugar concentration in nectar is a key determinant of flower quality for most pollinators, as nectar is a key source of energy. The second factor is the volume of nectar produced by different flowers; this has been seen to vary by at least two orders of magnitude. Some plants seem to barely produce any nectar at all (Hicks et al. 2016). Clearly plants that produce high sugar concentration nectar and lots of it are potentially going to be highly attractive to many pollinators. The protein composition of nectar can also be important, particularly for butterflies as this is their key source of adult nutrition. Nectar high in amino acids is believed therefore to be higher quality.
In the most extensive survey to date, a team at Edinburgh University (Hicks et al. 2016) found plants with daisy flowers (Asteraceae), to provide most of the biggest nectar rewards per blossom. In particular perennial yellow composites and thistles including several weed species were very good. However some non-daisies such as Malva moschata and Echium vulgare were also very good. Of course the exclusion of a species from one of these lists does not mean it is not valuable but simply that these lists of species reflect the assumptions and culture of the people running the study. In the Edinburgh study, for example, many of the species are native because it was undertaken by ecologists grounded in the belief that native species are good, but who also dabbled with some non-natives, the type of annuals found in pictorial meadows, because these were increasingly familiar in cities.

Our stress tolerant ‘steppe’ like experimental plantings at Green Estate, Sheffield. Photo: ©James Hitchmough
Our own studies at the University of Sheffield, on stress tolerant “steppe” like plantings of mainly near native species found, not surprisingly, that other types of flowers also offered highly valuable nectar rewards that were well used by pollinators. In particular, the near-native Origanum laevigatum and Eryngium planum were most outstanding at the whole plant level and Malva moschata (native), Stachys byzantina (near native), Potentilla nepalensis (non-native) are outstanding on a per flower basis. In contrast, Lychnis coronaria, another European near native species (listed as good on the Royal Horticulture Society perfect for pollinators list), appears to provide no nectar at all. So much for geography of origin, with regard to nectar. While other non-native species in our experiments, such as Oenothera tetragona and Penstemon barbatus were largely ignored by pollinators despite presenting big nectar rewards, P. barbatus is regularly visited by bees in my garden; many factors are at play in determining a plant’s value, including its context.
In more general terms, it seems from recent studies that perennial, as opposed to annual, herbaceous plants will, when not highly artificially altered, provide the most nectar. Those with large flowers provide more nectar per flower but less overall than those with masses of small flowers.
Pollen Rewards
Pollen is a particularly important resource for bees, who are pollen specialists, since they rear their larvae on it, but pollen is also utilized by hoverflies and flower-visiting beetles in their adult diets and is likely to be important for egg production in these groups.

Nectar and pollen accessibility: Salvia pratensis (meadow clary) with hidden pollen (black arrow) and nectar (white arrow) and Potentilla nepalensis (cinquefoil) with openly accessible nectar and pollen. Photo Salvia pratensis: ©Wikimedia Commons: K. Nell (2012); Photo Potentilla nepalensis: © Jack Brodie
Pollen quantities provided by different plants have been explored by Hicks et al. (2016) and are seen to vary widely by several magnitudes, from almost none produced in some plants to masses in others. In contrast to the situation with nectar, annual species appear to provide more pollen resources than perennials. In Hicks et al.’s (2016) study, the most pollen rich flowers were the annuals Escholtzia californica, Californian Poppy (non-native), and Papaver rhoes, Corn Poppies (widely seen as native but most likely an ancient introduced non-native from central Asia). The main gauge of pollen quality is protein content, which Roulston et al. (2000) found to vary widely, between species from 2.5% to 61%. Bees will collect low-quality pollen but it can detrimentally affect their health and larval development if too dominant in their diet. Whilst excellent for nectar, plants in the Asteraceae family typically have poor-quality pollen, whilst the pea family (Fabaceae) and Campanulaceae have high-quality pollen.
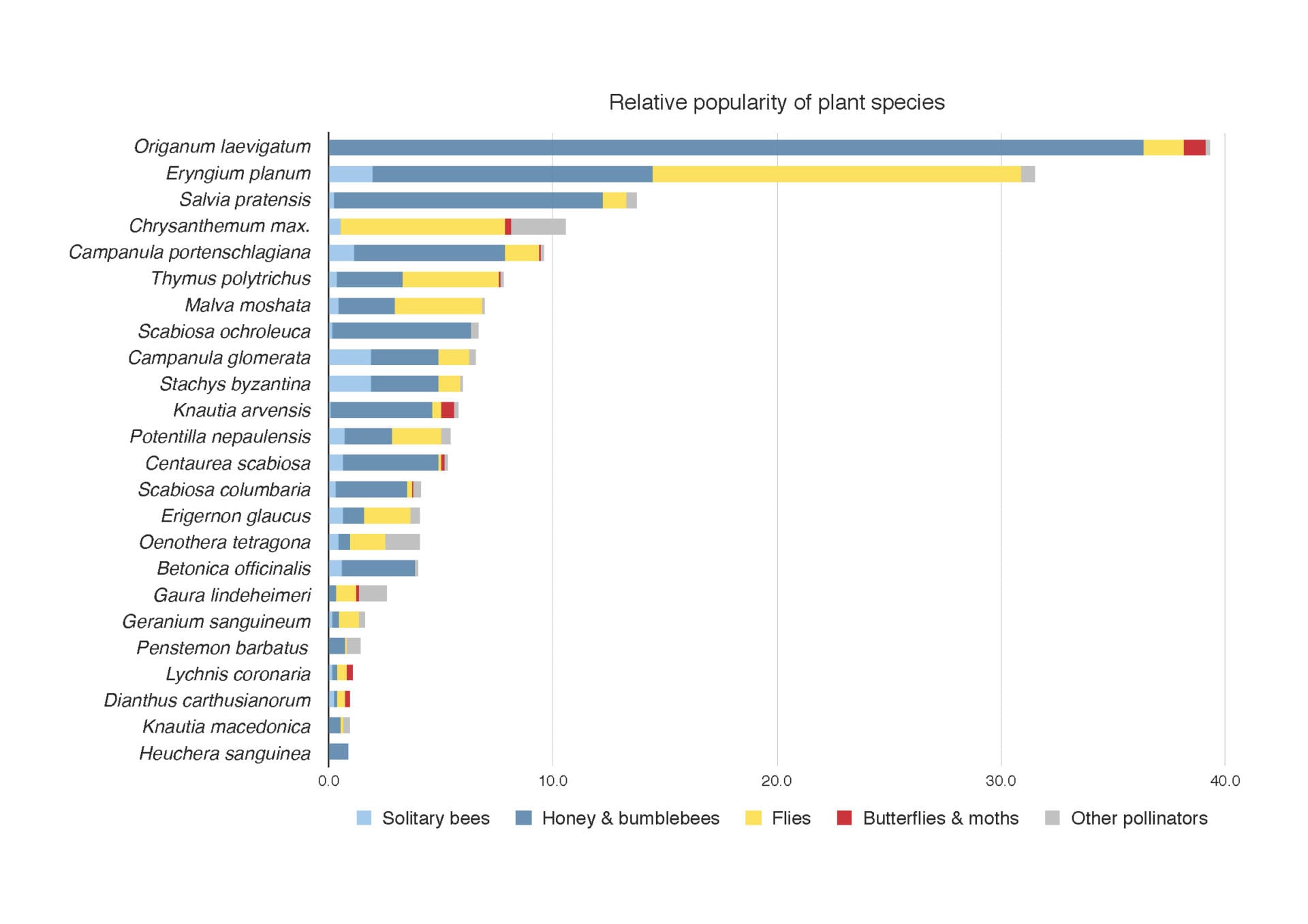
Popularity with pollinators of different plant species in our Sheffield study. ©Jack Brodie, James Hitchmough, and Michael Livingstone
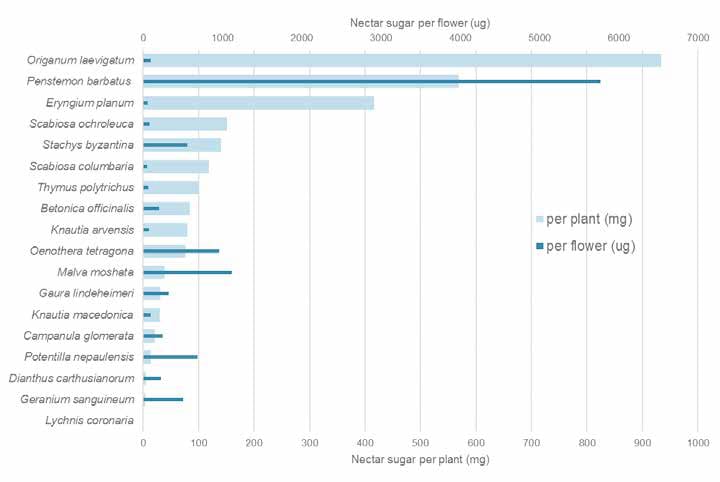
Nectar sugar resource provided by different plant species in our Sheffield study. ©Jack Brodie, James Hitchmough, and Michael Livingstone
Flower Morphology
One of the key specializing traits in a wide range of pollinators is the length of feeding mouthparts. If one selects plants with the nectaries at different depths within the flower, a diversity of pollinators can be supported. Deeply placed nectaries were preferred by bumble bees with longer tongues in our Sheffield field trials. Some complex-shaped flowers restrict access to both nectar and pollen rewards to exclude all pollinators other than certain bee species. Examples of these include many pea (Fabaceae) species such as lupins and vetches but also some Lamiaceae like Salvia pratensi, (meadow clary). In contrast to this, pollinators with less sophisticated or smaller feeding mouthparts, such as hoverflies, wasps, and some solitary bees, need flowers with more accessible floral nectaries and pollen such as is found in flatter flowers such as Potentilla nepalensis and shallower flowers such as Eryngium and other Apiaceae family members.

Near-native Stachys byzantina (lambs ear) is the favorite plant of the solitary wool carder bee (Anthidium maculatum). The plant provides both food and nesting materials. Photo: ©Jack Brodie
Flowering Times
The human desire to have flowers over as long a season as possible fits in well with supporting pollinators. Long-flowering species are potentially valuable because they help ensure continuity of supply. Early-flowering and late-flowering (autumn) species are also valuable to provide nectar and pollen when few plants are flowering in agricultural or semi-natural habitats. For example, bumble bee queens first emerge in early spring when they start their colonies; late in the season the new bumble bee queens and butterflies need to fatten up for hibernation. Non-native species are often particularly valuable at these times of year when few natives are in flower.
Putting These Principles into Practice
In most cases of multi-purpose/value, general, urban plantings, it will not be possible to base plantings purely on the most nectar- or pollen-rich species from the research we have highlighted. These species may not be appropriate either to the site, the aesthetic, and other functional requirements of the planting, or to the management regime. A degree of compromise is therefore inevitable, and in many cases desirable.

Native pollinators using non-native flowers: solitary leaf-cutter bee (Megachile centuncularis) on Non-native Erigeron glaucus (beach aster) was the most popular plant for leaf cutter bees in our study, possibly due to its accessible pollen. Photo: ©Jack Brodie
As we have discussed, species that are good for pollen may be poor for nectar and vice versa. We are likely to be most interested in supporting a diversity of pollinator species rather than massive populations of one species, for example lavender and honey bees. To do this, diversity of plant species is key, utilizing species that have medium to high sugar concentration and total volume of nectar, medium to high quality pollen, diverse floral forms, long flowering season, and diverse flowering times – from early spring to autumn. Diversity is also likely to be important in spatial terms at the scale of the plantings; having different communities in different spaces is probably a better strategy for pollinator diversity than repeating a seemingly premier pollinator community everywhere. This is precisely why domestic urban gardens are so successful in supporting exceptionally high native invertebrate diversity; every garden supports a significantly different plant flora. There is something somewhere for most invertebrates.
The scale of overall provision is also important; large areas of resource are ultimately able to support larger populations of pollinators than small patches can. At present the wealth of new scientific data on attractiveness of plants to pollinators is beginning to provide more clarity on how to use such information in design, but is also raising questions about how to provide access to this information and how to constantly update as new discoveries are made. One approach could be the development of an open access trait database along the lines of the German BIOFLOR project, but more design orientated. This could allow the maximization of the ecological benefit of landscape planting projects without restricting creative, bespoke planting design by having to rely on pre-designed off-the-shelf mixes or specialist expertise.
References
Baldock, K.C., Goddard, M.A., Hicks, D.M., Kunin, W.E., Mitschunas, N., Osgathorpe, L.M., Potts, S.G., Robertson, K.M., Scott, A.V., Stone, G.N. and Vaughan, I.P. (2015) Where is the UK’s pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. R. Soc. B, 282.
Baude, M., Kunin, W.E., Boatman, N.D., Conyers, S., Davies, N., Gillespie, M.A., Morton, R.D., Smart, S.M. and Memmott, J., (2016) Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature, 530.
Biesmeijer, J.C., Roberts, S.P.M., Reemer, M., Ohlemuller, R., Edwards, M., Peeters, T., Schaffers, A.P., Potts, S.G., Kleukers, R., Thomas, C.D., Settele J. and Kunin W. E. (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science, 313.
Garbuzov, M. and Ratnieks, F.L. (2014) Quantifying variation among garden plants in attractiveness to bees and other flower‐visiting insects. Functional Ecology, 28.
Hicks DM, Ouvrard P, Baldock KCR, Baude M, Goddard MA, Kunin WE, et al. (2016) Food for Pollinators: Quantifying the Nectar and Pollen Resources of Urban Flower Meadows. PLoS ONE, 11.
Roulston, T.H., Cane, J.H., Buchmann, S.L. (2000) What governs protein content of pollen: pollinator preferences, pollen–pistil interactions, or phylogeny? Ecological Monographs, 70.
Salisbury A, Armitage J, Bostock H, Perry J, Tatchell M, Thompson K. (2015) Enhancing gardens as habitats for flower-visiting aerial insects (pollinators): should we plant native or exotic species? Journal of Applied Ecology, 52.
Senapathi, D., Carvalheiro, L.G., Biesmeijer, J.C., Dodson, C.A., Evans, R.L., McKerchar, M., Morton, R.D., Moss, E.D., Roberts, S.P., Kunin, W.E. and Potts, S.G. (2015) The impact of over 80 years of land cover changes on bee and wasp pollinator communities in England. In Proc. R. Soc. B, 282.
Hanley ME, Awbi, AJ & Franco, M (2014) Going native? Flower use by bumblebees in English urban gardens. Annals of Botany,113.
About the Authors
Jack Brodie, James Hitchmough, and Michael Livingstone, Department of Landscape, University of Sheffield, U.K.
***
Each author appearing herein retains original copyright. Right to reproduce or disseminate all material herein, including to Columbia University Library’s CAUSEWAY Project, is otherwise reserved by ELA. Please contact ELA for permission to reprint.
Mention of products is not intended to constitute endorsement. Opinions expressed in this newsletter article do not necessarily represent those of ELA’s directors, staff, or members.



